Heat and Temperature are the words we use regularly, and we keep interchanging the meanings. Yes, they are different, but how? Why do we feel hot touching a cooker on a stove or chilly touching an ice block? We will see this in the article below.
What is Heat?
We define heat as the thermal energy that is transferred from one system to another.
- We call the body that is transferring this energy a hot body.
- The body that is receiving this energy is a cold body.
Therefore, heat is due to a transfer of energy between two systems.
Heat exists only when there is this movement of energy. The more the movement, higher is the thermal energy. A cup of coffee is hot, but we do not say it contains heat. We feel the heat when it is transferred to our hand when we touch it.
SI unit of heat if Joule (J). Another unit of heat is calories (cal).
What is Temperature?
Temperature is the degree of hotness or coldness of a body. While heat is the transfer of energy, the temperature is the ability of the body to transfer this heat.
- This means that a body or system with a higher temperature has a higher ability to transfer heat to the surrounding system.
- A body or system with a lower temperature has a higher ability to receive this energy.
SI unit of temperature is Kelvin (K). More common units are degree Celsius (oC) and degree Fahrenheit (oF).
Relationship between Heat and Temperature
Differences in temperature cause heat transfer from one system to another. Heat and temperature rise and fall together.
Heat (Q) and temperature (T) are related by the equation,
Q = mcΔT
where,
– m is the mass of the substance,
– c is the specific heat capacity,
– ΔT is the temperature change.
ΔT = Tfinal – Tinitial
If Tfinal > Tinitial, then there is an increase in temperature, and Q is positive. Thermal energy increases.
If Tfinal < Tinitial, then there is a decrease in temperature, and Q is negative. Thermal energy decreases.
Specific Heat Capacity
Specific heat capacity is the amount of heat required to increase the temperature of mass by 1 oC. Its unit is J/kg oC
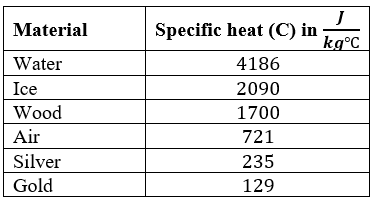
For example, to raise the temperature of 1 kg of water by 1 oC, 4186 Joules of heat energy is required.
For 1 kg of gold, just 129 J is enough.
We see that water is one of the substances having very high specific heat. This is important for maintaining life balance in nature.
Differences between Heat and Temperature
- Heat is a measure of the transfer of energy between the particles of the system.
- Heat depends on the amount of substance in a material (mass).
- A hot body transfers heat out from itself.
- A cold body receives heat from outside.
- Units of heat are Joules and Calories
- Heat is measured using a calorimeter.
- Temperature is a measure of the average kinetic energy of the particles in the system.
- Temperature does not depend on the mass.
- When transferred, temperature of a body reduces.
- When received, temperature of a body increases.
- Units of temperature are Kelvin, Celsius and Fahrenheit.
- Temperature is measured using a thermometer.
Methods of Transfer of Heat
We saw in the beginning that heat is due to the transfer of thermal energy between two systems. Heat transfer will take place only when there is a difference in temperature between the two systems. There are three ways in which heat is transferred:
a. Conduction
b. Convection
c. Radiation
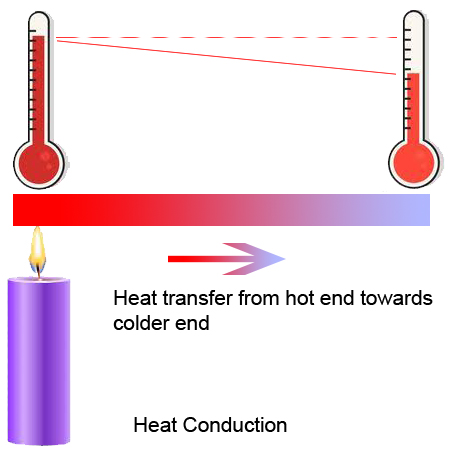
Conduction
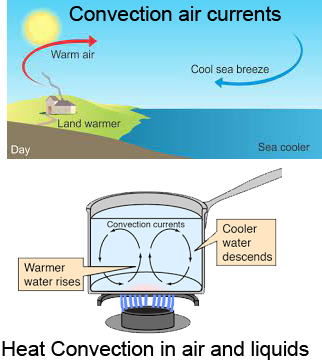
Convection
Radiation
- Heat transfer in solids
- Heat transferred between adjacent particles
- No physical movement of particles
- Examples: Hot vessel on a stove, melting wax in a candle, ironing of clothes
- Heat transfer in fluids
- Heat transferred by movement of fluid particles
- There is a physical movement of fluid particles
- Examples: Water boiling, ocean currents, the warmth of a fire
- Heat transfer by electromagnetic radiation
- It does not require any medium
There is no need for any medium - Examples: Heat from the sun, microwave oven
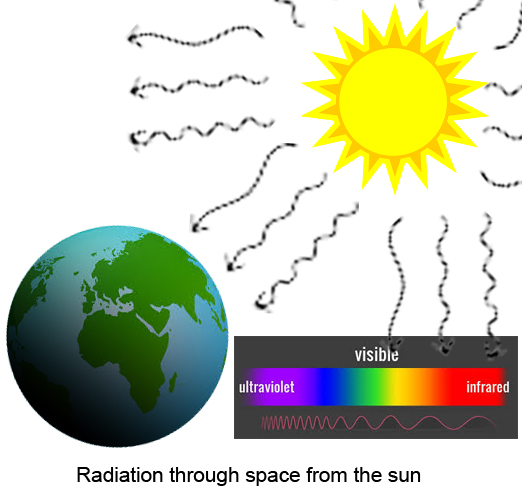
The infrared waves that are part of the electromagnetic spectrum are nothing but heat waves. These waves are longer in wavelength. Humans cannot see this wave that comes after visible light but can feel it on their skin as heat.
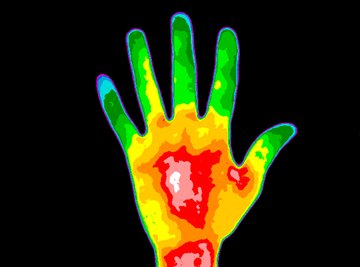
Thermal imaging binoculars use infrared radiation to “see” objects. All living beings radiate heat from their bodies. So, it is possible to make out living objects by the heat they emit.

Thermal Equilibrium
When two objects are at the same temperature, they are said to be in thermal equilibrium. The Zeroth Law of Thermodynamics says that no heat is transferred between two objects in thermal equilibrium. This law is applied in a thermometer. It reads the temperature when heat is transferred from the object to the mercury bulb/sensor of the thermometer. Only when the temperature of the thermometer equals that of the object, we get the reading.
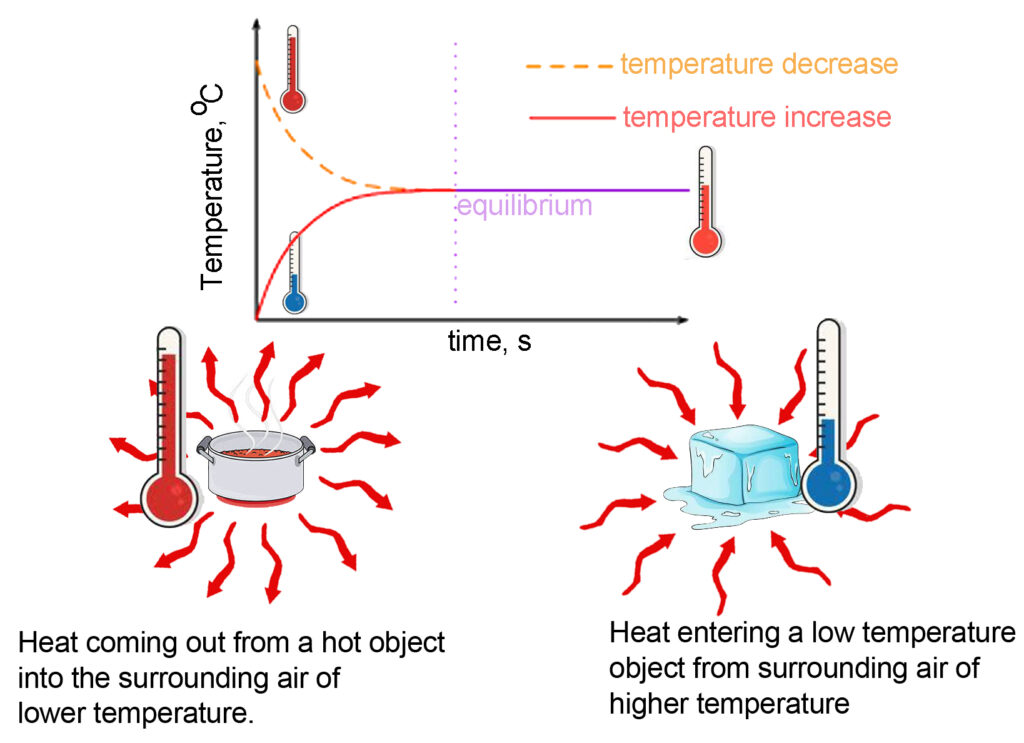
A hot cup of tea and an ice block are kept on a table. After some time, the teacup becomes cold. The ice melts.
a. The hot cup transfers its heat to the surrounding air of lower temperature, thereby reducing its own temperature.
b. The ice receives heat from the air, which has a higher temperature than itself. As the temperature of ice increases, its thermal energy increases and melts to become water
Do Cold Rays Exist?
Let us do a small experiment. On two ends of a long tube, keep two concave mirrors. At the focus of one, keep a hot object such as a candle. Place a thermometer at the focus of the second mirror. Notice that the thermometer registers a rise in the temperature. This shows that heat rays exist.
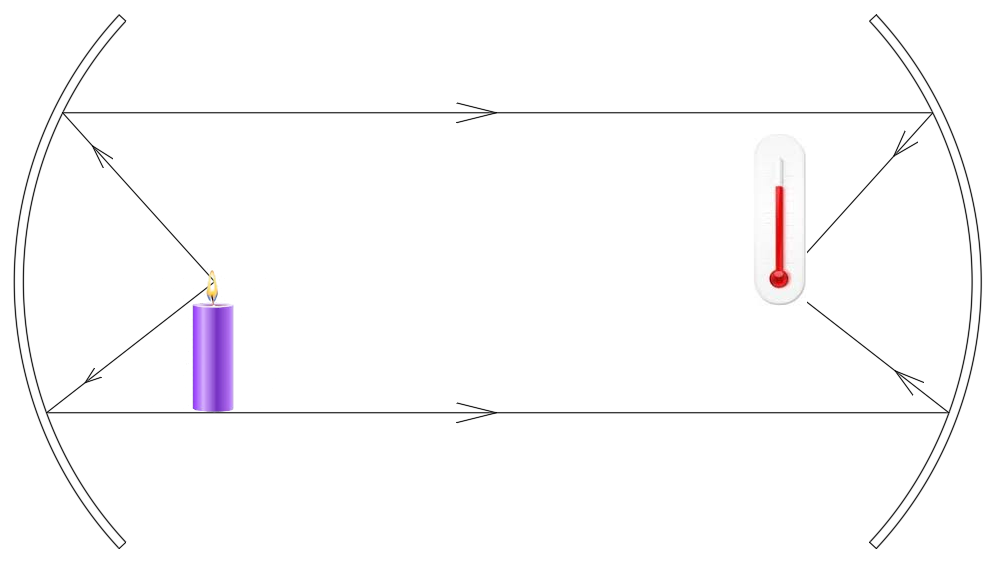
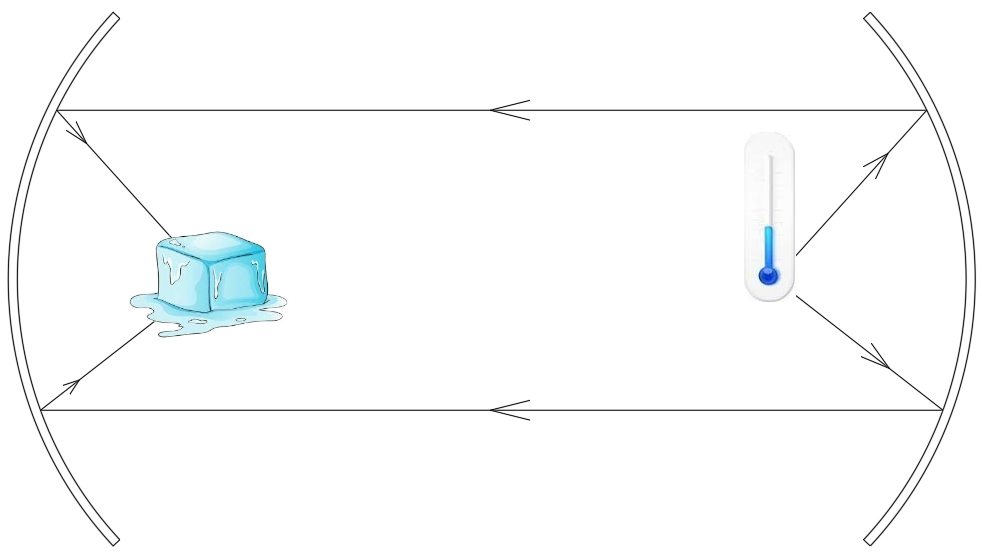
Now place a very cold object such as an ice block at the focus of one of the mirrors. Notice that the thermometer registers a decrease in temperature.
Does it mean that cold rays from the ice block are transferred to the thermometer to make it show a lesser temperature? Does this mean cold rays exist, just like hot rays?
Notice the direction of arrows in the second case. Actually, heat rays from the thermometer are being transferred to the ice block, and not the reverse. Compared to the ice, the thermometer was at a higher temperature, so the ice was absorbing its heat by reflecting through the mirrors. As the heat of the thermometer is being sucked by the ice, the thermometer loses its heat, showing a dip in its recording.
This means that there is no such thing as a cold ray, and cold rays do not exist in nature.
Some Trivia
- Thermos flasks have two containers separated by a space with a vacuum. Heat transfer by conduction or convection needs a medium. They cannot happen in a vacuum, unlike radiation. There is no way for the heat to go out or in. Therefore, the things in a thermos flask stay at the same temperature for a long time.
- We wear warm clothes in winter. Warm clothes do not create heat. They are made of materials that are bad conductors of heat. Actually, it is our body that is creating heat, warm clothes prevent the heat of our body from escaping out into the air of lower temperature.
- Coldness means low temperature. Our body is at a higher temperature than ice. Due to the vast gap in temperature, it quickly absorbs the heat of our hands. So, we suddenly feel very cold when we touch ice.
- The lowest temperature possible in the universe is -273.15 oC. It is 0 Kelvin. Temperature cannot go below this.
- The handles of kitchen vessels, cookers are made of plastic. Plastic or wooden spatula is used while cooking. These are bad conductors of heat. They do not get hot quickly.


All these days, I thought heat and temperature were same. Got to know they are different from your excellent article. More needed.
Today, I went to the beach with my kids. I found a sea shell and gave it to my 4 year old daughter and said “You can hear the ocean if you put this to your ear.” She placed the shell to her ear and screamed. There was a hermit crab inside and it pinched her ear. She never wants to go back! LoL I know this is totally off topic but I had to tell someone!
Hi there! This post couldn’t be written any better! Reading through this post reminds me of my previous room mate! He always kept talking about this. I will forward this article to him. Pretty sure he will have a good read. Thank you for sharing!
Thanks again for the article.Really looking forward to read more. Keep writing.